The launch of our Virus Hunter diagnostics platform is to get a further boost this month with a trial at Royal Lancaster Infirmary. The Virus Hunter (VH6) RT-LAMP testing device that has been developed in collaboration with Lancaster University, Brunel University London and the University of Surrey is now being used to test NHS patients on COVID wards.
Dr Muhammad Munir, Lecturer in Biomedicine at Lancaster University who is leading the trial has said “This clinically validated device will enhance the community testing capacity of the UK, and globally. This will save costs, and will provide fast track diagnostics, resulting in early case detection, thus promoting effective disease management and preventing community spread, and ultimately saving lives.” He also added “Once the clinical validation testing of the devices has been completed, the device will pave the way for global distribution.”

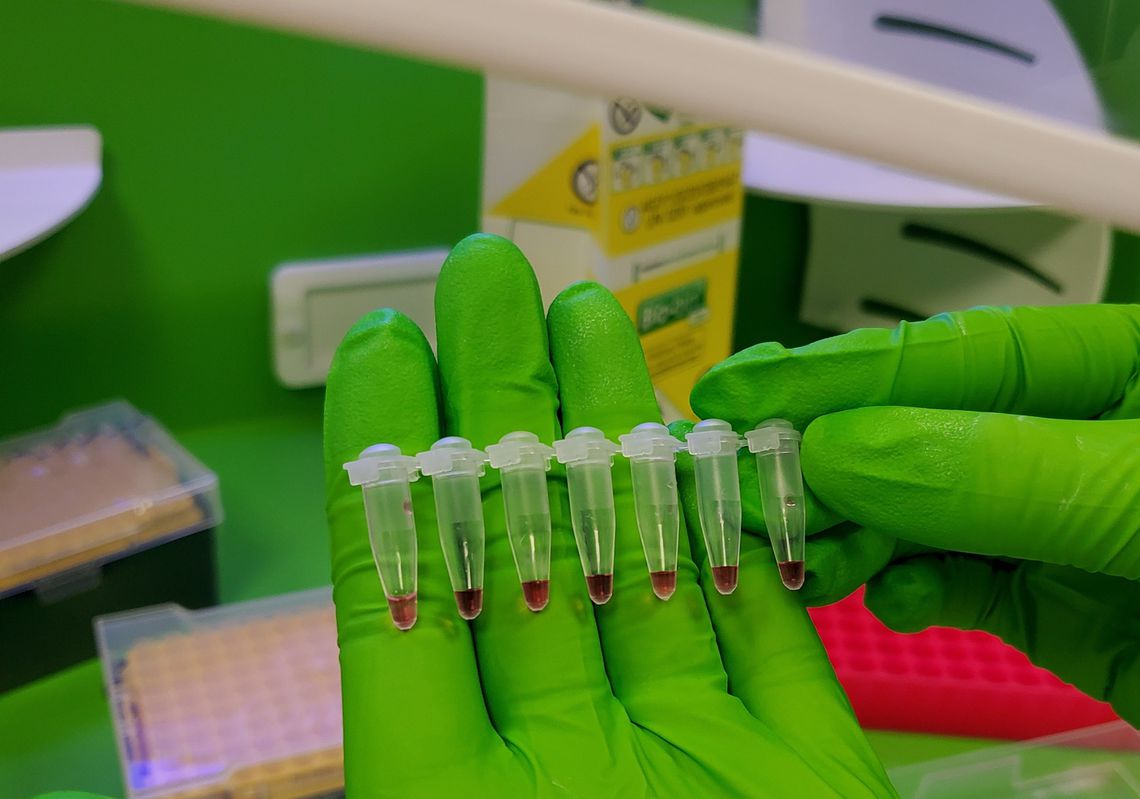
The Virus Hunter diagnostic platform takes patient nasal and oral samples and uses RT-LAMP technology to identify the presence of SARS-CoV-2 and can test up to 6 samples simultaneously, giving results within 20 minutes. Post the NHS validation this will enable the device to be deployed in multiple locations around the country and further afield where ongoing mass testing is required.
Our CEO David Rimer said: “The team at Vidiia are extremely grateful to Dr Munir and his team at Lancaster University for heading up our first NHS trial. By gaining more results using NHS patient samples across three COVID wards, this will assist hugely in generating significantly more data from positive patients across a broader demographic. This is yet another great example of how effective our consortium of universities is in their valued contribution to the success of a product that allows our customers to operate with less restrictions and ultimately assist in a strengthening economic outlook.”
One part of the collaborative project involves Lancaster University and Morecambe Bay University Hospitals NHS Trust who will collect samples from each patient admitted to the Royal Lancaster Infirmary.

Dr Craig Williams is Consultant Microbiologist from University Hospitals of Morecambe Bay NHS Foundation Trust and has said in a Lancaster University press release this week: "We are delighted to be involved in this trial of a new, rapid, test for COVID-19 which builds on our collaboration in providing testing for our local population early in the pandemic. It is another step forward to increasing community testing not only here in the UK, but around the world. We are privileged to be playing a crucial role in the trial and look forward to following its progress."
Professor Wamadeva Balachandran at Brunel University London, whose team were instrumental in the early development of the test device has said "the trial will further validate VH6, not only in the developed world, but in less developed countries and remote areas because it doesn't need expensive support equipment and health workers need little training to use it”.
The NHS trial in Lancashire is being carried out whilst testing of students and staff continues on campus at Surrey University. Roberto La Ragione, Professor of Veterinary Microbiology and Pathology at the University of Surrey has said: “This trial, together with the validation testing that is taking place on our campus, brings us potentially one step closer to the rolling out of this device on a national and global scale. From the beginning of this pandemic there has been a pressing need to develop, an accurate, inexpensive, rapid device that can be used to diagnose COVID-19. Initial results from our testing with Virus Hunter 6 has shown that it has the potential to do just this.”
Our diagnostic device has already received approval from the Medicines and Healthcare products Regulatory Agency and has CE marking. It can test up to six samples simultaneously and was shown to be 99 per cent accurate in lab trials. It is currently being used to focus on COVID19, but developments are already underway to introduce new assays to test for other global viral infections.
For all enquiries please email sales@vidiia.co.uk
